 In Regulating E-Cigarettes, No Easy Fix For The FDA – Health Affairs Blog
In Regulating E-Cigarettes, No Easy Fix For The FDA – Health Affairs Blog:

by Prof. Wendy Parmet //Northeastern University School of Law
Sometime in the next few months, the Food and Drug Administration (FDA) is expected to issue the so-called deeming regulations, which will open the door to the federal regulation of e-cigarettes. In considering whether to issue the regulations, which were first published for notice and comment rulemaking last April, the FDA faces a formidable challenge: it must decide whether and how to regulate in the midst of scientific uncertainty and limited statutory flexibility. By subjecting e-cigarettes to its regulatory regime, the FDA risks retarding the growth of what may prove to be a powerful new tool for harm reduction. But by failing to act, the agency risks undermining decades of progress in tobacco control. In either case, the public health impact is apt to be significant.
It is a victory that was made possible, in large measure, by a constellation of state and federal regulatory interventions: laws regulating the marketing and sale of cigarettes, barring sales to youth, banning indoor smoking, and taxing cigarette sales have all played a role in reducing rates of smoking.
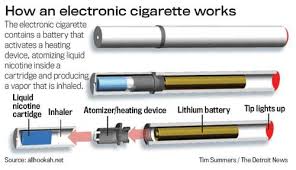
Nevertheless, smoking remains stubbornly common, especially among teens. According to the Centers for Disease Control, in 2012 an estimated 18.1 percent of the U.S. adults were smokers as were 14 percent of high school students. Each year over 480,000 Americans die of smoking-related diseases. It is in this environment that the market for e-cigarettes has developed since they were first introduced in the U.S. less than ten years ago. By 2013, over 47 percent of smokers had tried e-cigarettes; and 4 percent were regular users. In 2013 more than 250,000 minors who had never smoked had used an e-cigarette. Although they take many forms, e-cigarettes, also known as electronic nicotine delivery systems (ENDS), or more colloquially as vapes, use battery power to heat and vaporize liquid nicotine that users inhale. In contrast to traditional cigarettes, pipes, and cigars, there is no combustion.
Supporters contend that e-cigarettes can serve as a form of harm reduction, akin to the needle exchange programs that have been used to stop the spread of HIV by intravenous drug use. Less dangerous than cigarettes, e-cigarettes deliver nicotine without exposing users and bystanders to the byproducts of combustion. Therefore they may allow users to get their nicotine “fix” and the sensory pleasures of smoking without being exposed to, or exposing others to, cigarette smoke.
Critics, in contrast, argue that e-cigarettes are not as harmless as they seem. For a start, they expose users to nicotine, a highly addictive drug, which has been associated with numerous health effects. Moreover, studies have shown that the vapor produced by e-cigarettes sometimes contain heavy metals and other toxins.
For example a recent letter to the editor of the New England Journal of Medicine reported on research finding that high voltage vaping may expose users to more than 4 times the amount of formaldehyde presented by traditional cigarettes. And because there are over 400 brands on the market, and most of them are imported, the risk of adulteration is high, as a recent New York Times article pointed out. Critics also worry that the growth of vaping will lead to more smoking. In particular, they point to the common practice of adding sweet flavors to e-cigarettes and argue that this attracts young people, who may become addicted to nicotine and then “graduate” to conventional cigarettes. There is also concern that the advertising and use of e-cigarettes in public places will renormalize smoking, thereby reversing the change in social norms that has helped support reductions in rates of cigarette usage.
Regulatory Challenges
The challenge for regulators is that the science is not yet settled. In its notice of proposed rulemaking,the FDA admitted that it did “not currently have sufficient data about these products to determine what effects e-cigarettes have on the public health.” Although the weight of the evidence establishes that e-cigarettes are less harmful than cigarettes, it is simply too early to know the long-term dangers for users or bystanders. Moreover, we don’t know if the proliferation of e-cigarettes will increase or reduce overall rates of smoking. In other words, we just don’t know whether e-cigarettes will prove to be an effective form of harm reduction or a gateway drug.
Adding to the regulatory challenge are the limitations imposed by the federal statutory scheme. In 2010 a federal appeals court ruled that the FDA could not act against adulterated e-cigarettes under the Food, Drug and Cosmetic Act; rather its authority was limited to that provided by the 2009 Tobacco Control Act(“TCA”). That Act imposes regulations on specified tobacco products, such as cigarettes and smokeless tobacco, and all other tobacco products that the FDA “deems” to be subject to the Act. It is this provision that the FDA will invoke if it promulgates the deeming regulations. 'via Blog this'








.jpg)